Phosphorus
Luxury uptake of phosphorus is a biological treatment process whereby the bacteria usually found in the activated sludge treatment portion of the secondary wastewater treatment plant are withdrawn to an environment without oxygen (anaerobic). When the bacteria are faced with the situation of apparent death, the bacteria release phosphorus from their cell structure in large quantities. Phosphorus can then be removed and disposed of by using lime for settling similar to the previously discussed lime clarification process. After the bacteria have released their phosphorus, they are placed back into an ideal environment with oxygen and food. In this environment, since the bacteria are lacking in phosphorus in their cell structure, the first thing they take in is phosphorus. This phosphorus take-up is known as luxury uptake and is used in the process for biological removal of the phosphorus within the wastewater treatment facilities.
Bacteria found in normal activated sludge process use phosphorus within the makeup of the cell structure that forms the bacteria. When the bacteria are in a state of Endogenous respiration or are very hungry and need food and oxygen they tend to absorb phosphorus quite freely. This process is called luxury uptake in which the bacteria take excess phosphorus into their bodies due to the stimulation of being placed in a proper environment containing food and oxygen. When these same bacteria are placed in an environment where there is no oxygen (anaerobic), the first element that is released by the bacteria as they begin to die is phosphorus. As the phosphorus is released, it can be drawn off and removed from the wastewater stream.
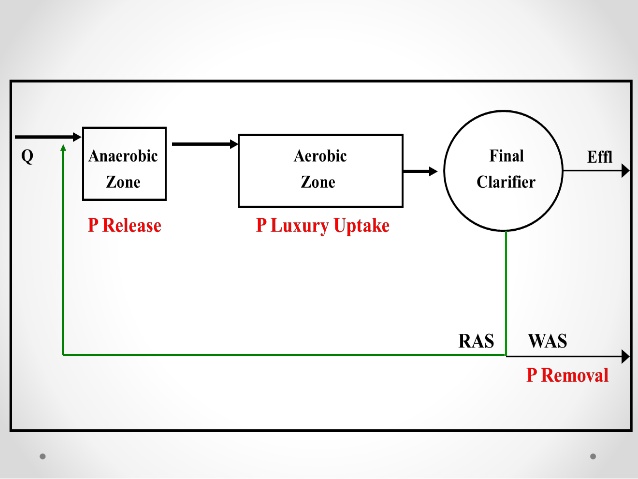
Luxury uptake of phosphorus is found at activated sludge treatment plants. The units used include the standard ones for an activated sludge plant plus a relatively deep detention basin where anaerobic conditions exists.
Another unit commonly found in the luxury uptake and removal system is a lime clarification tank (clarifier) which is usually capable of treating 10 percent of the wastewater flow stream through the treatment facility. Return pumps and piping continue to move the activated sludge through an anaerobic state to a phosphorus release point and back to aeration for the luxury uptake process to begin all over.
Because luxury uptake can only take place in a very controlled environment, the bacteria cannot be exposed to any condition which would prevent them from either taking up phosphorus into their cell structure or releasing the phosphorus at the proper time. The basic operation requires the operators to remove the activated sludge from the secondary clarifier and provide the proper detention time in an anaerobic tank for the release to the phosphorus trapped within the cell structure of the bacteria. The operator must closely regulate the time of the anaerobic condition. The bacteria should not be allowed to die. However, the length of time should be sufficient to remove as much of the phosphorus as possible. Return the activated sludge to an area of the aeration tank where sufficient oxygen and primary effluent exist so that the bacteria can be revived and can take up the maximum amount of phosphorus within their cell structure.
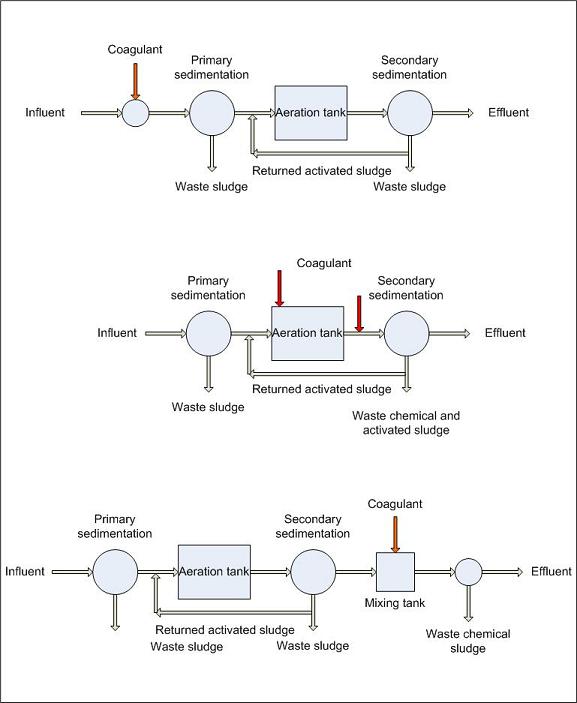
Aluminum sulfate (alum) in combination with wastewater also can flocculate phosphorus in much the same way as lime precipitation. The flocculation that happens with aluminum sulfate addition is the formation of aluminum phosphate particles that attach themselves to one another and become heavy and settle to the bottom of a clarifier. The aluminum sulfate and phosphorus mixture can then be withdrawn, thereby removing the phosphate or phosphorus from the wastewater flow. This alum floc is difficult to settle out in a clarifier. Therefore, a sand or mixed-media filter is usually placed after the clarifier to remove the remaining floc.
Aluminum sulfate (alum) can be used in the same manner as lime for precipitation of phosphorus in a clarifier. The same principles of coagulation, flocculation and sedimentation apply when using alum for the removal of phosphorus in effluent from a secondary treatment facility. However, because of the difference in cost between aluminum sulfate and lime, lime is more commonly used for the precipitation of phosphorus. When alum is used for phosphorus removal, two general reactions occur. In the first reaction, alum reacts with the alkalinity of the wastewater to form an aluminum hydroxide floc.
Phosphorus removal is by the formation of an insoluble complex precipitate and by adsorption on the aluminum hydroxide floc. Depending on the alkalinity of the wastewater, dosages of 200 to 400 mg/l of alum are commonly required to reduce phosphorus in the effluent down to 0 to 0.5 mg/l. Optimum phosphorus removal is usually achieved around a pH of 6.0. Alum feed is frequently controlled by automatic pH equipment which doses according to the pH set point (the more alum, the lower the pH). Jar tests can be used to determine the optimum pH set point and alum dosage rate.
If it is necessary to achieve low effluent phosphorus residuals (less than 1.0 mg/l) in the effluent, the chemical clarifier is usually followed by either a pressure filter or a multi-media gravity filter. Phosphorus sludge from the clarifier goes to dewatering and disposal facilities. At present, there are no economical methods available for alum recovery.